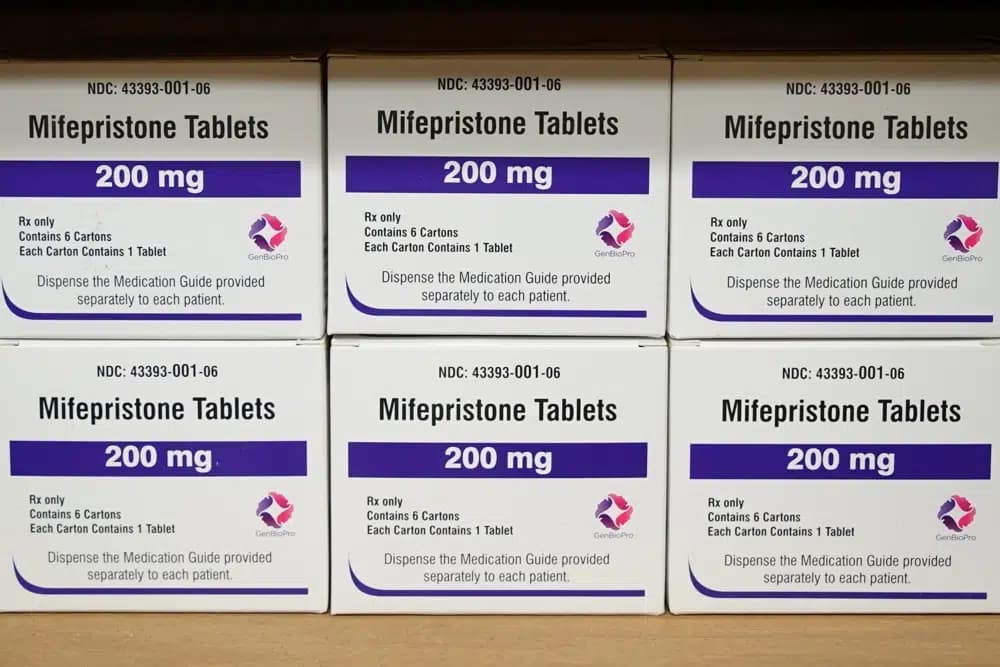
NEW YORK – After the federal government greenlighted the sale of abortion pills at retail pharmacies nationwide, the U.S. Bishops Conference pro-life committee chair has said that mothers in need “deserve better.”
“We decry the continuing push for the destruction of innocent human lives and the loosening of vital safety standards for vulnerable women,” said Bishop Michael Burbidge of Arlington, the USCCB pro-life chair, in a statement.
“This week’s action by the FDA not only advances the obvious tragedy of taking the lives of the preborn, but is also harmful to women in need,” Burbidge said.
The decision was made by the Food and Drug Administration, an agency within the U.S. Department of Health and Human Services earlier this week. There was no news release or formal announcement of the change. However, the FDA updated its website on January 3 to reflect it.
The change relates to mifepristone, the first pill in a two-step medication abortion regimen. The drug, which the FDA authorizes to be used in the first 10 weeks of pregnancy, was approved in 2000. The second drug in the regimen, misoprostol, which is used to treat other medical conditions also, can be easily obtained at pharmacies through the typical prescription process.
Essentially, the change makes the drug similar to other prescription medications, both in-person at a retail pharmacy or through the mail. Patients still need to get a prescription from a certified healthcare provider to obtain the drug.
The F.D.A.’s January 3 change follows a 2021 review of the Mifepristone Risk Evaluation and Mitigation Strategy (REMS), which found that the data supported a “modification of the REMS to reduce the burden on the health care delivery system and to ensure the benefits of the product outweigh the risks.”
Under the change, retail pharmacies can dispense mifepristone so long as they complete a certification process. The FDA also removed the requirement that patients must obtain the abortion pill in-person.
A 2020 report from the Guttmacher institute, a research organization that supports abortion rights, found that more than half of all abortions that year were medical abortions. Discussions on medical abortion access and regulations have grown since, especially after the Supreme Court overturned the federal right to an abortion in a ruling last year.
Most abortions are now banned in at least 13 states.
Burbidge highlighted data from the Charlotte Lozier Institute, a pro-life research organization, showing the increased risks of medical abortion compared to surgical abortion. The data states that medical abortion has a complication rate four times that of surgical abortion, and as many as one out of five women will suffer a complication.
“Overturning the safety protocols around abortion-causing drugs to effectively make them available on demand at pharmacies, requiring no in-person medical supervision, facilitates the isolation of critically vulnerable pregnant women, and invites more risk, pain and trauma,” Burbidge said.
After the FDA announcement, Walgreens and CVS – the two largest drugstore chains in the U.S. – indicated they would begin the certification process to be able to sell mifepristone. The two companies cannot offer the pill in states that have implemented abortion bans.
Burbidge expressed concern for what the FDA regulation changes mean for pharmaceutical workers who may have conscientious objections to selling the drugs.
“[The changes] may also result in new violations of conscience for pharmacy workers who cannot dispense such drugs,” Burbidge said. “The FDA should protect the life and health of both mothers and children, not loosen safety standards under industry or political pressures.”
“We call on the administration to correct its policy priorities and stand with mothers in need,” he added. “They deserve better.”
Follow John Lavenburg on Twitter: @johnlavenburg